Prospects for Transcranial Magnetic Stimulation in Epilepsy
Alexander Rotenberg, M.D., Ph.D.
Co-Director, Animal Behavior and Physiology Core
Director, Neuromodulation Program
November 16, 2021
Transcranial magnetic stimulation (TMS) is a method for focal noninvasive cortical stimulation where small intracranial electrical currents are induced by a strong extracranial magnetic field. TMS falls into the broad category of “neuromodulation” protocols that include a range of invasive and noninvasive methods for delivering electrical signals to the brain and spinal cord. However, unlike most brain stimulation techniques that are deployed for treatment in drug-resistant disorders, TMS stands out as a method with as much diagnostic as therapeutic capacity.
DIAGNOSTIC TMS APPLICATIONS
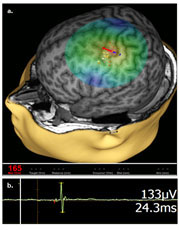
When applied over the motor cortex, TMS reliably induces a motor evoked potential (MEP; Figure 1) that can be recorded in a contralateral limb muscle by surface electromyography (EMG). TMS-EMG is in fact the most common use of TMS as a diagnostic tool. That is, by stimulating the motor cortex and recording the MEP, and operator can: (1) map the motor cortical representation, and (2) generate an in vivo input-output curve to define cortical excitability. Both of these diagnostic TMS applications are now in use in our clinical epilepsy program.
 Motor mapping by TMS-EMG (by an FDA-cleared device), has enabled our epilepsy group to identify functional motor cortical areas in cases where the anatomy of a seizure onset zone may overlap with the motor cortex. Notably, for young children with epilepsy, who are unable to cooperate with functional MRI, TMS-EMG is the only option to obtain a clear motor map and to test whether a planned surgery will compromise motor function. (A related protocol for language mapping by TMS enables our clinical team to answer the same question when the anatomy of the seizure onset zone may overlap with cortical language areas.)
Motor mapping by TMS-EMG (by an FDA-cleared device), has enabled our epilepsy group to identify functional motor cortical areas in cases where the anatomy of a seizure onset zone may overlap with the motor cortex. Notably, for young children with epilepsy, who are unable to cooperate with functional MRI, TMS-EMG is the only option to obtain a clear motor map and to test whether a planned surgery will compromise motor function. (A related protocol for language mapping by TMS enables our clinical team to answer the same question when the anatomy of the seizure onset zone may overlap with cortical language areas.)
- TMS-EMG measures of cortical excitability, a “stress test” designed to measure cortical excitability, while not FDA-cleared as a diagnostic procedure, has also emerged in recent years as a practical research tool in epilepsy. TMS-EMG metrics such as threshold to MEP activation, MEP amplitude and MEP modulation by a range of paired-pulse TMS-EMG paradigms enable quantification of the excitation:inhibition (E:I) balance, which is abnormal in most epilepsies and can be normalized by interventions ranging from ani-seizure drugs to disease-modifying gene therapies.
At time of this writing, ongoing studies at BCH include measures of E:I in Dravet syndromes, CDKL5 deficiency, succinic semialdehyde dehydrogenase deficiency, a range of focal drug-resistant epilepsies, and syndromes, such as autism, where epilepsy is common and the E:I may be abnormal.
THERAPEUTIC APPLICATIONS
As an anti-epileptic therapy, repetitive TMS (rTMS) protocols, where hundreds of consecutive stimuli are delivered to a single cortical region, has realistic potential as a means to access use-dependent synaptic plasticity and suppress excess cortical excitability. rTMS, li
ke other forms of neuromodulation can be deployed in the ictal (in-seizure) or interictal (between seizure) states to either interrupt an ongoing prolonged seizure or to prevent the next seizure from starting. Our group and others have deployed rTMS in cases of drug resistant epilepsy into which a large minority, about 1/3 of patients with epilepsy fall.
From the translational neuroscience perspective, the wide clinical use of rTMS offers opportunities to develop and rapidly test novel anti-seizure protocols. Since rTMS devices are abundant in the clinical arena (they are used widely to treat pharmacoresistant major depression) and have a favorable safety profile, anti-seizure rTMS protocols that are developed in the preclinical space can rapidly translate to clinical trials. Among such realistic prospects is identification of stimulation patterns that are optimized for seizure suppression, and logical rTMS-drug coupling that can improve the rTMS potential for seizure suppression.
TMS IN THE ANIMAL BEHAVIOR AND PHYSIOLOGY CORE
In the Animal Behavior and Physiology Core (AB&P) of our IDDRC, we have a unique rat TMS setup where human rTMS and TMS-EMG protocols can be effectively mimicked. IDDRC users now have access to preclinical TMS experiments aimed to test changes in cortical excitability that follow drug administration. The threshold question of whether an agent designed to modulate cortical excitability has done so can be addressed by TMS in our AB&B setup. Further, the magnitude of such effect as a function of dose or time after delivery can be measured for purposes of defining pharmacodynamics of a range of CNS-active drugs.
Since TMS can be safely done in rats and in humans, with similar readouts, the utility of TMS-derived translational biomarkers has become apparent to industry and academia groups to whom our services are available. We are here to help and to advise.
Visit us:
Animal Behavior and Physiology Core (AB&P)
Intellectual and Developmental Disabilities Center
Boston Children’s Hospital